Which Best Symbolizes the Hydrogen Bonding Between Two Water Molecules
D Question 8 3 pts Which covalent bond is the most polar. Hydrogen bonding causes water to remain liquid over a wide temperature range.
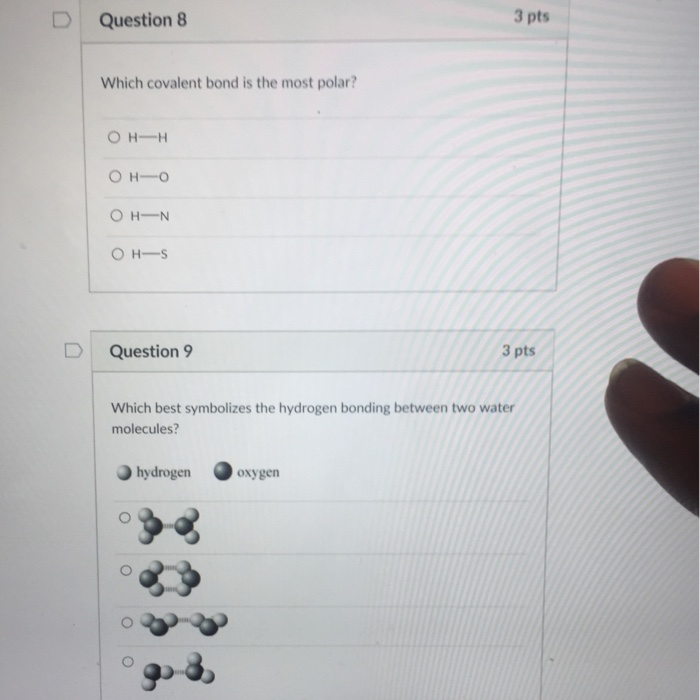
Solved D Question 8 3 Pts Which Covalent Bond Is The Most Chegg Com
They become a liquid.

. A polar. B the bond between O of one water molecule and H of a second water molecule. A different state of matter.
But because of the hydrogen bonds as water molecules come together they stick to one another for a small but significant amount of time. The four electron pairs surrounding the oxygen tend to arrange themselves as far from. 35 The hydrogen bond between two water molecules forms because water is A polarB nonpolar.
Weak moderate and strong bonds with energetic boundaries at about 2 and 15 kcalmol. The most stable arrangement is the. Hydrogen bonding forms in liquid water as the hydrogen atoms of one water molecule are attracted towards the oxygen atom of a n.
A is a base. 35 The hydrogen bond between two water molecules forms because water is. The weak hydrogen bonds involve less polar X-H groups in proton donors like C-H or.
Causes stickiness between water molecules when hydrogen on one water is attracted to oxygen on another. A gas is a physical state of matter where the molecules are far apart and moving very quickly. It all comes down to electronegativity and polarity.
C is an acid. Which best represents the intermolecular attractions between the two molecules. 38 Which of the following is an example of hydrogen bonding.
Hydrogen bonding is a special type of dipole-dipole attraction between molecules not a covalent bond to a hydrogen atom. Water is an excellent example of hydrogen bonding. All of the electron pairsshared and unsharedrepel each other.
Answer 1 of 4. Question 30 2 points Possible interactions between a water molecule and formaldehyde are shown below. D Question 8 3.
There is a partial negative charge near the oxygen atom and partial positive charges near the hydrogen atoms due to the uneven distribution of electrons between the atoms which results in the formation of. In water each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. - is a measure of an atoms attraction for an electron in a chemical bond.
The electronegativity difference for each bond is as. The bond is between the hydrogen of one water molecule and the oxygen atoms of another water molecule not between the two hydrogen atoms a common misconception. The hydrogen bond length of water varies with temperature and pressure.
How this works is that the polar nature of the water molecule means each. Water molecules are polar covalent molecules. This is because the oxygen atom in addition to forming bonds with the hydrogen atoms also carries two pairs of unshared electrons.
- Is the total negative charge on a polyatomic anion. The hydrogen bond in water is a dynamic attraction between neighboring water molecules involving one hydrogen atom located between the two oxygen atoms. Based on this information milk of magnesia.
Due to the electronegativity difference between the atom pairs mentioned electrons are unevenly shared across the covalent bond. - metallic element reacts with a nonmetallic element. Hydrogen bonding is an attractive force between two molecules that relies on the slight polarity of the O-H O-F or O-N bond.
This attraction leaves the hydrogen nucleus with a partial positive charge. A 025 M aqueous solution of potassium chloride KCl is tested for conductivity using the type of apparatus shown. A water molecule consists of two hydrogen atoms bonded to an oxygen atom and its overall structure is bent.
At the same time the oxygen end of the other water molecule carries a partial negative charge. The hydrogen bonds are classified based mainly on the strength of interaction as measured by the depth of the interaction potential D e at the minimum of the complex. Which best symbolizes the hydrogen bonding between two water molecules.
- decreases from left to right across a period on the periodic table. Water H 2 O. This slows them down and holds them closer to one another.
B is hydrophobic. A hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded to a small highly electronegative atom is attracted to a lone pair of electrons on an atom in a neighboring molecule. In H 2 O only two of the six outer-shell electrons of oxygen are used for this purpose leaving four electrons which are organized into two non-bonding pairs.
Chemistry questions and answers. D Question 9 3 pts Which best symbolizes the hydrogen bonding between two water molecules. Hydrogen Bonding and Water Molecules.
Even though a hydrogen bond is only 5 as strong as a covalent bond its enough to stabilize water molecules. What do you predict will happen. As it takes extra energy to break hydrogen bonds water has an unusually high heat of vaporization.
D a small molecule. B the bond between O of one water molecule and H of a second water molecule. As hydrogen bond strength depends almost linearly on its length shorter length giving stronger.
Usually three classes are distinguished. Examples of Hydrogen Bonds. Water has a much higher boiling point than other hydrides.
- is the same for all of the elements in a family or group. When a hydrogen bond forms between two water molecules the hydrogen atom of one water molecule is attracted to the oxygen of the other water molecule. Although the mean H-OO angle is indicated as 12 it varies over a wide angle considering both the quantum nature of the nuclei and thermal fluctuations with 50 probability between 4 and 24 at 250 K.
What is the interaction between water and formaldehyde called. 0-H E van der Waals hydrogen bonding ion-dipole London Dispersion forces induced dipole. Hydrogen bond strengths range from 4 kJ to 50 kJ.
A force between two different molecules. Hydrogen bonds are very strong compared to other dipole interactions. It results from the attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as a N O or F atom and another very electronegative atom.
The strength of a typical hydrogen bond is about 5.

Chem 100 Exam 2 Connect Flashcards Quizlet

Chem 100 Exam 2 Ch 8 Flashcards Quizlet

No comments for "Which Best Symbolizes the Hydrogen Bonding Between Two Water Molecules"
Post a Comment